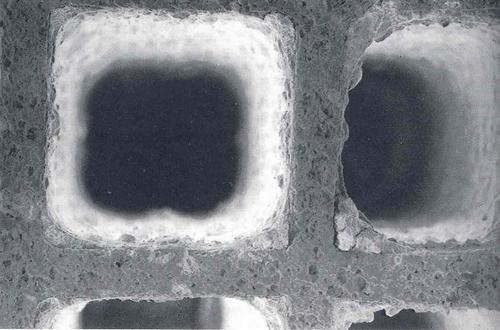
DPF structure
Diesel Particulate Filters (DPFs) remove particulates from diesel engines through physical filtration. Among the various types of filters available, the ceramic monolith (cordierite or carborundum) with a honeycomb structure is the most widely used.
DPFs are similar in design to catalytic converter inserts, featuring intersecting honeycomb channels. However, DPF inserts have a larger diameter and porous walls whose inlets and outlets are coated with a catalytic layer that serves as a base for catalytic metal particles. World HYDRO FLUSH specializes in advanced cleaning solutions for such filters with efficient removal of soot and particulates but preserves the integrity of the filter structure.
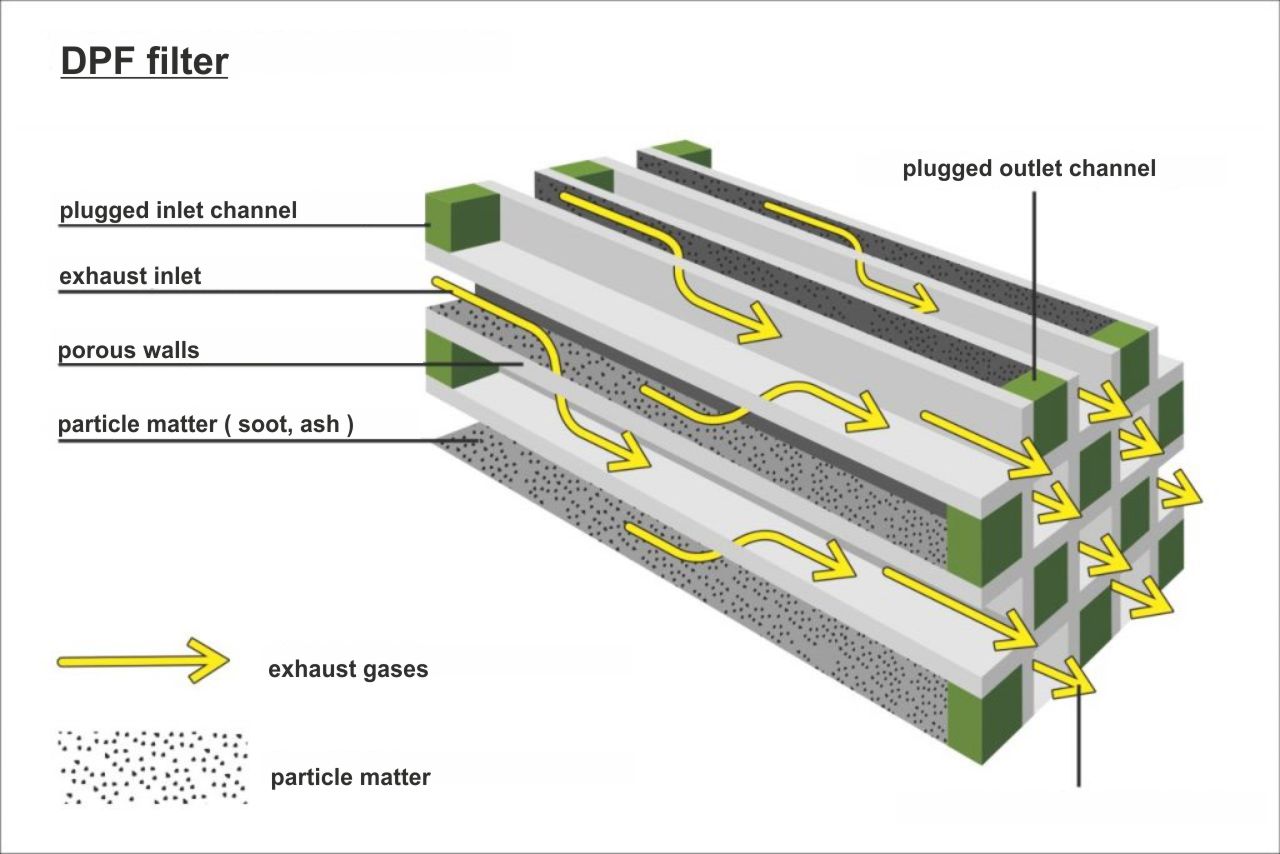
The channels blocked on the outlet side are called inlet channels – these are where exhaust fumes get into the filter. And the channels with closed ends on the inlet side are outlet channels where exhaust fumes get out.
Wanting to go through such an obstacle, they have to get through the pores leaving larger particles trapped inside the blinded channels.
Monoliths
Made of cordierite, carborundum or aluminum titanate.
The walls of the filter insert (monolith) have the arrangement of tiny pores which are carefully controlled in the production process. The total porosity of the material is usually from 40% to 50% or more, whereas the average sizes of pores are usually 10 to 20 μm. The mechanism of filtration in monolithic wall filters is the combination of the filtration of penetration and clumping soot. The penetration filtration is a prevailing mechanism as particulates deposit in the network of pores inside the wall material. With an increase in the soot accumulation, a layer of particles forms along the wall surface in inlet channels, and then the filtration of the soot clumping becomes a prevailing mechanism. As a rule, monolithic filters have the filtration capacity between 70 and 95% of all particulates.
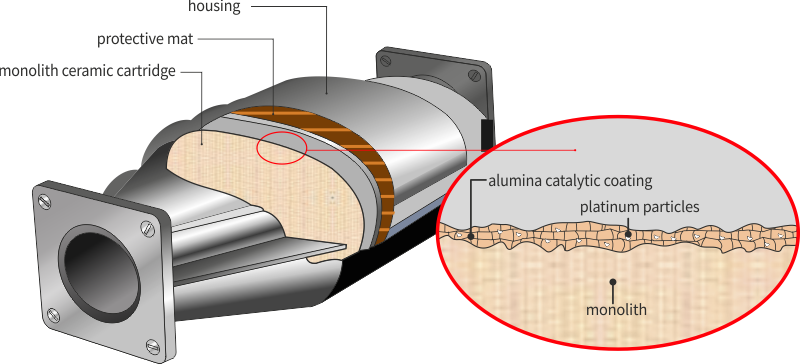
Catalytic coating
The main function of the catalytic coating is to ensure the substrate for catalytic (precious) metals. Moreover, the catalytic coating can physically separate and prevent unwanted reactions between the components of the complex catalytic system.
The catalytic coating materials include inorganic base metal oxides, such as alumina, silicon monoxide, cerium oxide, titanium dioxide, zirconium monoxide and zeolites.
Some of them are used as catalysts supports, others are added to the catalyst coating as promoters or stabilizers, still others reveal catalytic activity.
Good materials of the catalytic coating are characterized by high thermal stability.
The catalytic coating is applied on the monolith of water-based suspension.

Catalytic (precious) metals
The catalyst of (y) precious metals can be present in the catalyst coating suspension, or they are used in the second stage called impregnation. During the impregnation, the monolith coated with the catalytic coating is immersed in aqueous solution comprising catalytic precursors. A bit dried catalyst is dried and calcined to the final form. During calcination the catalyst precursors decompose forming the final catalyst, usually a metal or a metal oxide. The most common catalysts are the platinum group metals (PGM), such as platinum itself (Pt), palladium (Pd) and rhodium (Rh).
Ceramic mat
It is wrapped around the monolith. It provides thermal insulation, protection against mechanical shocks and the vehicle vibrations.